Background: Signaling through the mTOR pathway contributes to growth, progression and chemoresistance of several cancers. Accordingly, inhibitors have been developed as potentially valuable therapeutics. Their optimal development requires consideration of dose, regimen, biomarkers and a rationale for their use in combination with other agents. Using the infrastructure of the Comparative Oncology Trials Consortium many of these complex questions were asked within a relevant population of dogs with osteosarcoma to inform the development of mTOR inhibitors for future use in pediatric osteosarcoma patients.
Methodology/principal findings: This prospective dose escalation study of a parenteral formulation of rapamycin sought to define a safe, pharmacokinetically relevant, and pharmacodynamically active dose of rapamycin in dogs with appendicular osteosarcoma. Dogs entered into dose cohorts consisting of 3 dogs/cohort. Dogs underwent a pre-treatment tumor biopsy and collection of baseline PBMC. Dogs received a single intramuscular dose of rapamycin and underwent 48-hour whole blood pharmacokinetic sampling. Additionally, daily intramuscular doses of rapamycin were administered for 7 days with blood rapamycin trough levels collected on Day 8, 9 and 15. At Day 8 post-treatment collection of tumor and PBMC were obtained. No maximally tolerated dose of rapamycin was attained through escalation to the maximal planned dose of 0.08 mg/kg (2.5 mg/30 kg dog). Pharmacokinetic analysis revealed a dose-dependent exposure. In all cohorts modulation of the mTOR pathway in tumor and PBMC (pS6RP/S6RP) was demonstrated. No change in pAKT/AKT was seen in tumor samples following rapamycin therapy.
Conclusions/significance: Rapamycin may be safely administered to dogs and can yield therapeutic exposures. Modulation pS6RP/S6RP in tumor tissue and PBMCs was not dependent on dose. Results from this study confirm that the dog may be included in the translational development of rapamycin and potentially other mTOR inhibitors. Ongoing studies of rapamycin in dogs will define optimal schedules for their use in cancer and evaluate the role of rapamycin use in the setting of minimal residual disease.
Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs
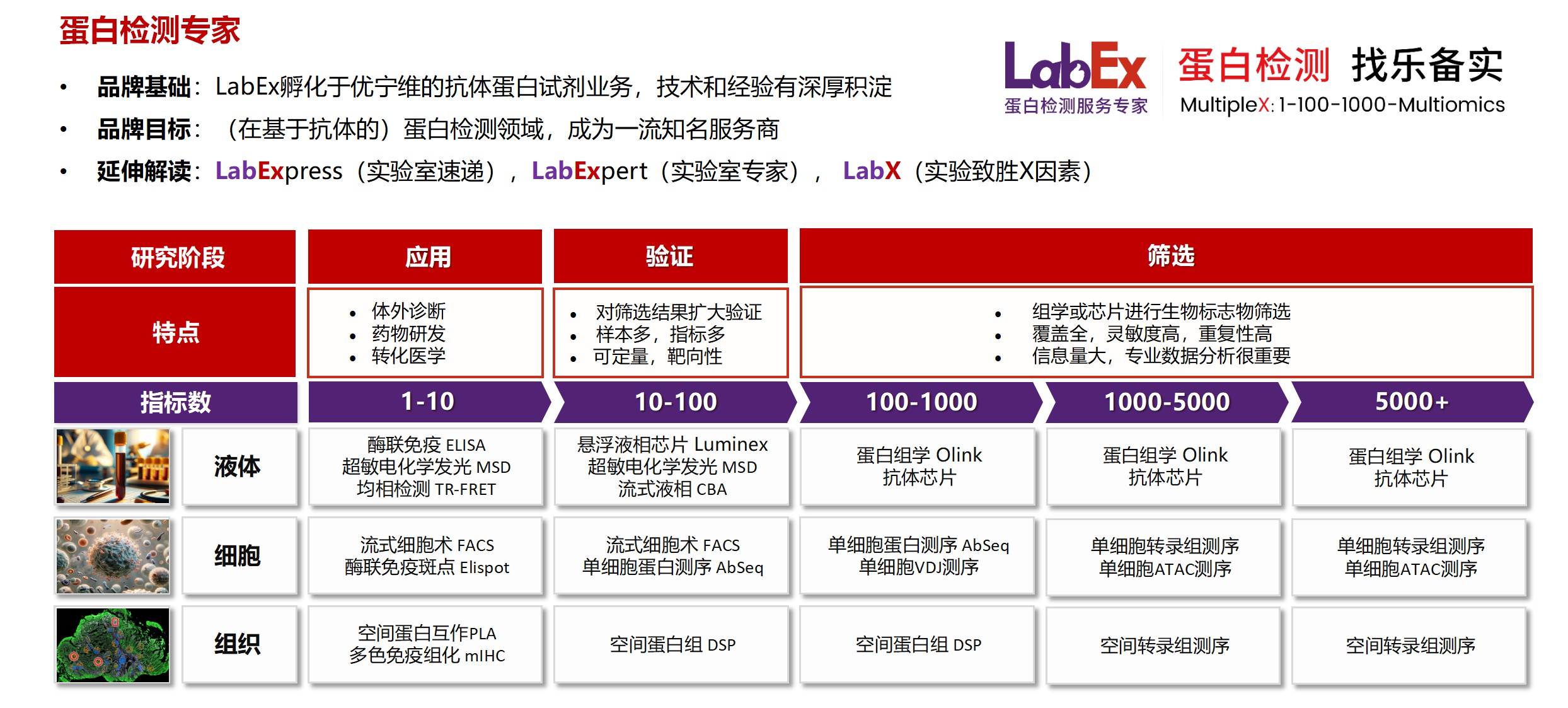
乐备实是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


