Background: Overproduction of proinflammatory cytokines from activated microglia has been implicated as an important contributor to pathophysiology progression in both acute and chronic neurodegenerative diseases. Therefore, it is critical to elucidate intracellular signaling pathways that are significant contributors to cytokine overproduction in microglia exposed to specific stressors, especially pathways amenable to drug interventions. The serine/threonine protein kinase p38α MAPK is a key enzyme in the parallel and convergent intracellular signaling pathways involved in stressor-induced production of IL-1β and TNFα in peripheral tissues, and is a drug development target for peripheral inflammatory diseases. However, much less is known about the quantitative importance of microglial p38α MAPK in stressor-induced cytokine overproduction, or the potential of microglial p38α MAPK to be a druggable target for CNS disorders. Therefore, we examined the contribution of microglial p38αMAPK to cytokine up-regulation, with a focus on the potential to suppress the cytokine increase by inhibition of the kinase with pharmacological or genetic approaches.
Methods: The microglial cytokine response to TLR ligands 2/3/4/7/8/9 or to Aβ1-42 was tested in the presence of a CNS-penetrant p38α MAPK inhibitor, MW01-2-069A-SRM. Primary microglia from mice genetically deficient in p38α MAPK were used to further establish a linkage between microglia p38α MAPK and cytokine overproduction. The in vivo significance was determined by p38α MAPK inhibitor treatment in a LPS-induced model of acute neuroinflammation.
Results: Increased IL-1β and TNFα production by the BV-2 microglial cell line and by primary microglia cultures was inhibited in a concentration-dependent manner by the p38α MAPK-targeted inhibitor. Cellular target engagement was demonstrated by the accompanying decrease in the phosphorylation state of two p38α MAPK protein substrates, MK2 and MSK1. Consistent with the pharmacological findings, microglia from p38α-deficient mice showed a diminished cytokine response to LPS. Further, oral administration of the inhibitor blocked the increase of IL-1β in the cerebral cortex of mice stressed by intraperitoneal injection of LPS.
Conclusion: The p38α MAPK pathway is an important contributor to the increased microglial production of proinflammatory cytokines induced by diverse stressors. The results also indicate the feasibility of targeting p38α MAPK to modulate CNS proinflammatory cytokine overproduction.
Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ)
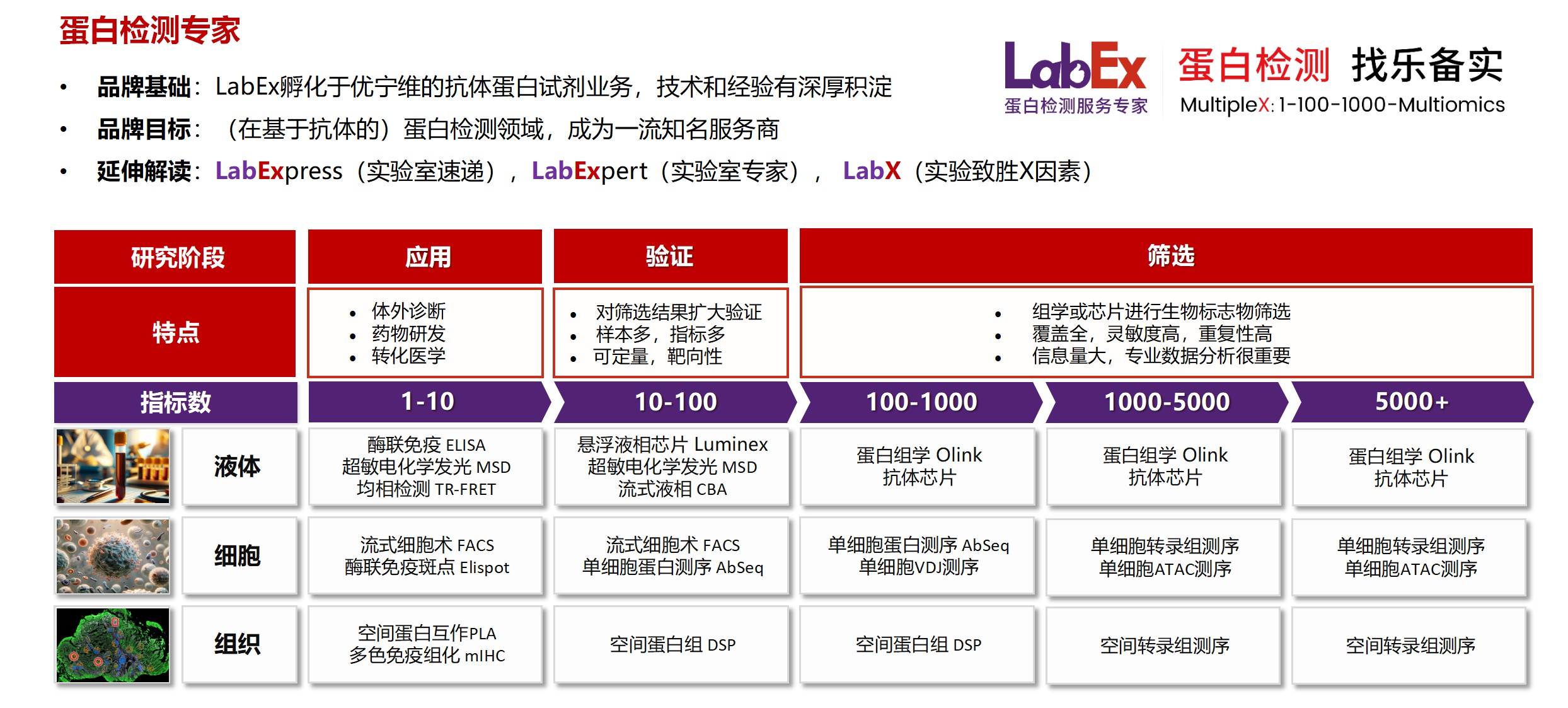
乐备实(上海优宁维生物科技股份有限公司旗下全资子公司),是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


