Safety, Efficacy, and Biomarker Exploration in a Phase II Study of Bevacizumab, Oxaliplatin, and Gemcitabine in Recurrent Müllerian Carcinoma
OBJECTIVE: To explore the safety, efficacy, and biomarkers of bevacizumab with gemcitabine and oxaliplatin in women with recurrent platinum-sensitive ovarian carcinoma. METHODS: The patients received bevacizumab (10 mg/kg), gemcitabine (1000 mg/m(2)), and oxaliplatin (65 mg/m(2)) on days 1 and 15 in 28-day cycles. The patients with safely accessible tumor underwent intratumoral fluid pressure (IFP) measurements and positron-emission tomographies immediately and 2 weeks after treatment. Blood biomarkers were evaluated at 5 time points. RESULTS: The trial was closed after enrolling 19 of the 53 projected patient accrual. Thirteen (68.5%) of 19 patients showed a response (1 complete response, 12 partial responses), and 6 patients showed stable disease (31.6%). Median progressive-free survival was 36.9 weeks (258.3 days), and the median overall survival was 112.3 weeks (633 days, not reached). Toxicity was acceptable, and there were no arterial thromboses, serious bleeding, gastrointestinal perforations, or complications from the invasive procedures. Bevacizumab with chemotherapy induced a substantial drop in tumor IFP after treatment. The regimen induced sustained elevation in circulating plasma vascular endothelial growth factor (VEGF), placental growth factor (PlGF), basic fibroblast growth factor (bFGF), soluable vascular endothelial growth factor receptor 2 (sVEGFR2), and circulating progenitor cells. Plasma PlGF, VEGFR2(+) monocytes, and urinary matrix metalloproteinase 2 activity showed differential associations with treatment outcome when evaluated at baseline and after 14 days of treatment. CONCLUSIONS: Despite early termination of the study, the results indicate that the regimen was well tolerated and demonstrated activity in platinum-sensitive ovarian cancer. Biomarker evaluations showed that bevacizumab with chemotherapy significantly changed the levels of several circulating cellular and molecular biomarkers. The increases in plasma PlGF and VEGFR2(+) monocytes showed correlations with outcome. These exploratory data should be further evaluated in future studies of bevacizumab in ovarian cancer.
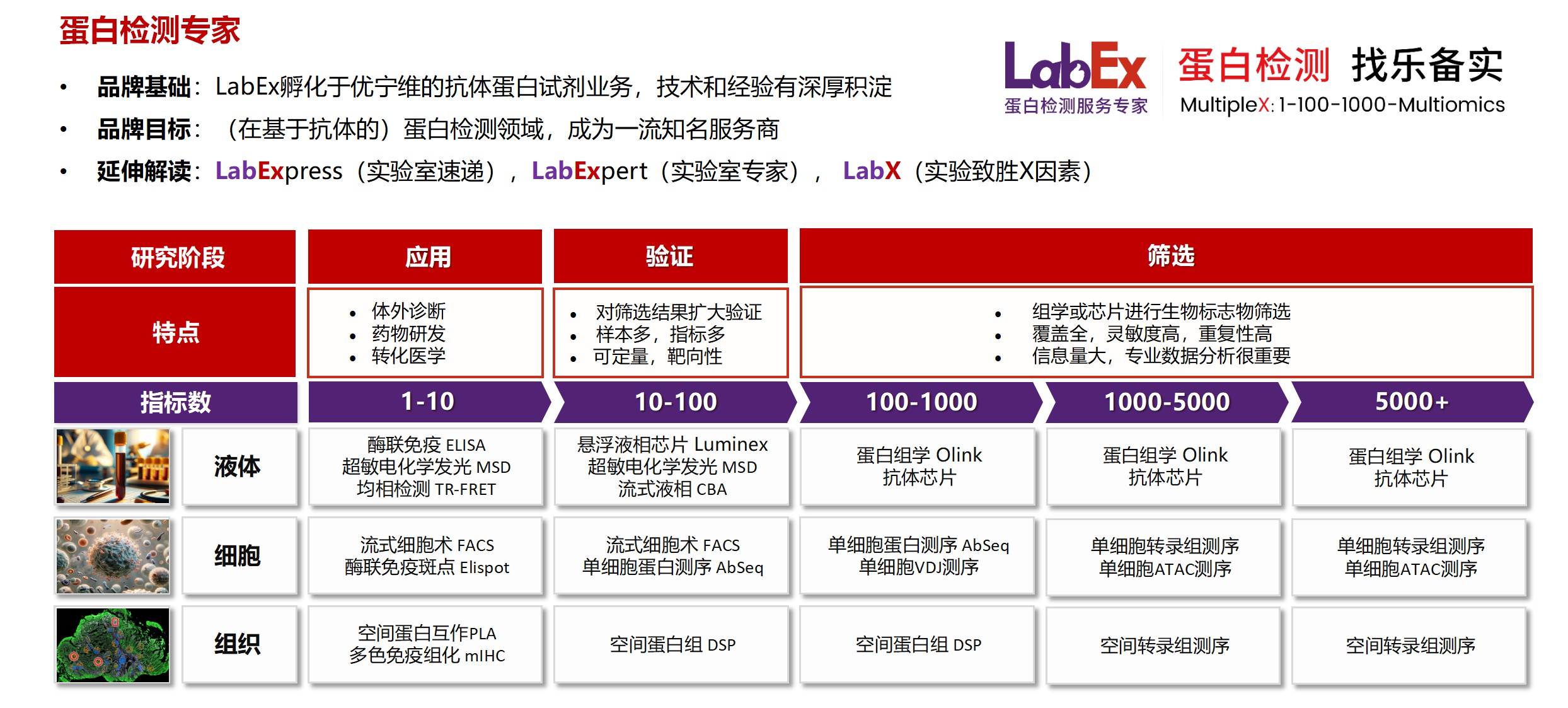
乐备实是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


