Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach
Annual outbreaks of influenza infections, caused by new influenza virus subtypes and high incidences of zoonosis, make seasonal influenza one of the most unpredictable and serious health threats worldwide. Currently available vaccines, though the main prevention strategy, can neither efficiently be adapted to new circulating virus subtypes nor provide high amounts to meet the global demand fast enough. New influenza vaccines quickly adapted to current virus strains are needed. In the present study we investigated the local toxicity and capacity of a new inhalable influenza vaccine to induce an antigen-specific recall response at the site of virus entry in human precision-cut lung slices (PCLS). This new vaccine combines recombinant H1N1 influenza hemagglutinin (HAC1), produced in tobacco plants, and a silica nanoparticle (NP)-based drug delivery system. We found no local cellular toxicity of the vaccine within applicable concentrations. However higher concentrations of NP (≥10(3) µg/ml) dose-dependently decreased viability of human PCLS. Furthermore NP, not the protein, provoked a dose-dependent induction of TNF-α and IL-1β, indicating adjuvant properties of silica. In contrast, we found an antigen-specific induction of the T cell proliferation and differentiation cytokine, IL-2, compared to baseline level (152±49 pg/mg vs. 22±5 pg/mg), which could not be seen for the NP alone. Additionally, treatment with 10 µg/ml HAC1 caused a 6-times higher secretion of IFN-γ compared to baseline (602±307 pg/mg vs. 97±51 pg/mg). This antigen-induced IFN-γ secretion was further boosted by the adjuvant effect of silica NP for the formulated vaccine to a 12-fold increase (97±51 pg/mg vs. 1226±535 pg/mg). Thus we were able to show that the plant-produced vaccine induced an adequate innate immune response and re-activated an established antigen-specific T cell response within a non-toxic range in human PCLS at the site of virus entry.
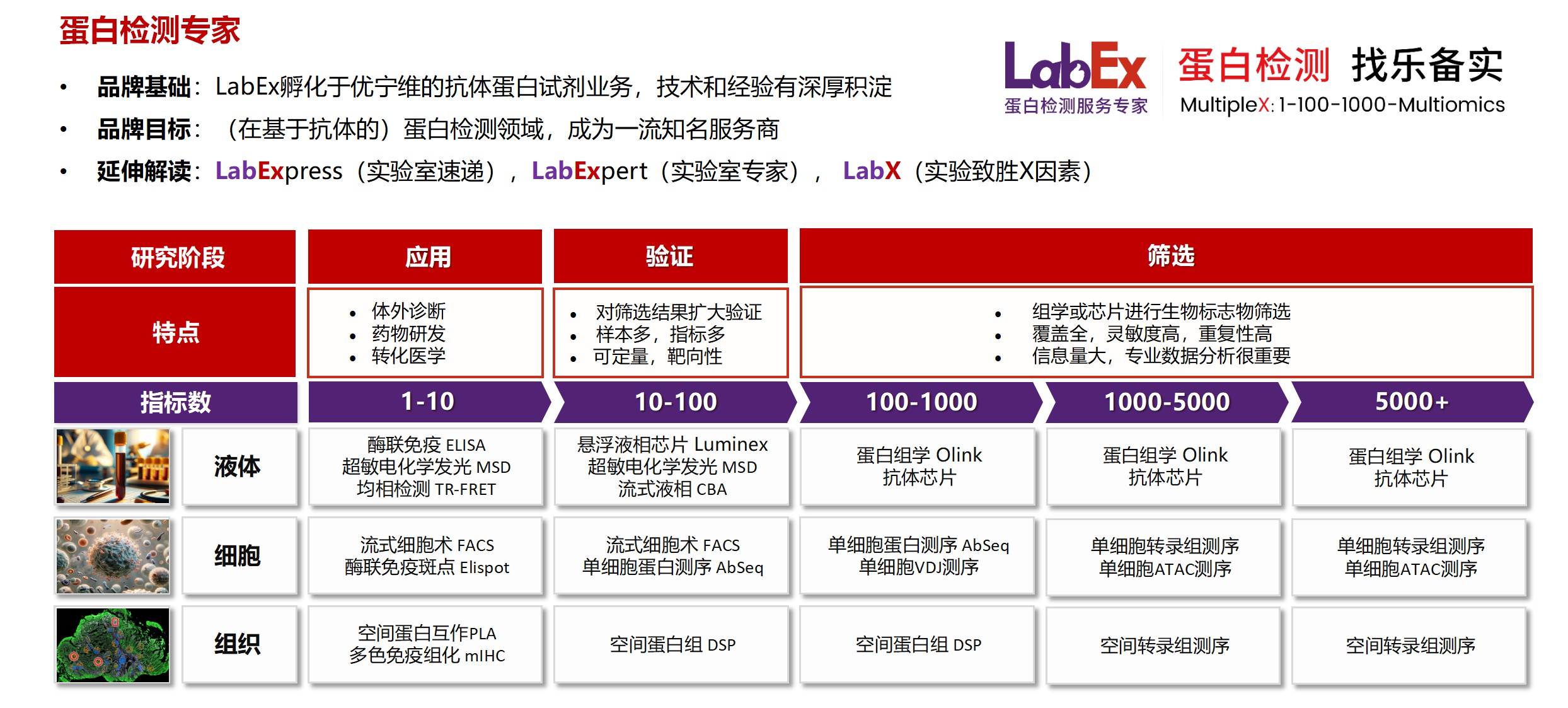
乐备实是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


