Aims/hypothesis: Ingested protein is a well-recognised stimulus for glucagon-like peptide-1 (GLP-1) release from intestinal L cells. This study aimed to characterise the molecular mechanisms employed by L cells to detect oligopeptides.
Methods: GLP-1 secretion from murine primary colonic cultures and Ca(2+) dynamics in L cells were monitored in response to peptones and dipeptides. L cells were identified and purified based on their cell-specific expression of the fluorescent protein Venus, using GLU-Venus transgenic mice. Pharmacological tools and knockout mice were used to characterise candidate sensory pathways identified by expression analysis.
Results: GLP-1 secretion was triggered by peptones and di-/tripeptides, including the non-metabolisable glycine-sarcosine (Gly-Sar). Two sensory mechanisms involving peptide transporter-1 (PEPT1) and the calcium-sensing receptor (CaSR) were distinguishable. Responses to Gly-Sar (10 mmol/l) were abolished in the absence of extracellular Ca(2+) or by the L-type calcium-channel blocker nifedipine (10 μmol/l) and were PEPT1-dependent, as demonstrated by their sensitivity to pH and 4-aminomethylbenzoic acid and the finding of impaired responses in tissue from Pept1 (also known as Slc15a1) knockout mice. Peptone (5 mg/ml)-stimulated Ca(2+) responses were insensitive to nifedipine but were blocked by antagonists of CaSR. Peptone-stimulated GLP-1 secretion was not impaired in mice lacking the putative peptide-responsive receptor lysophosphatidic acid receptor 5 (LPAR5; also known as GPR92/93).
Conclusions/interpretation: Oligopeptides stimulate GLP-1 secretion through PEPT1-dependent electrogenic uptake and activation of CaSR. Both pathways are highly expressed in native L cells, and likely contribute to the ability of ingested protein to elevate plasma GLP-1 levels. Targeting nutrient-sensing pathways in L cells could be used to mobilise endogenous GLP-1 stores in humans, and could mimic some of the metabolic benefits of bariatric surgery.
Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor
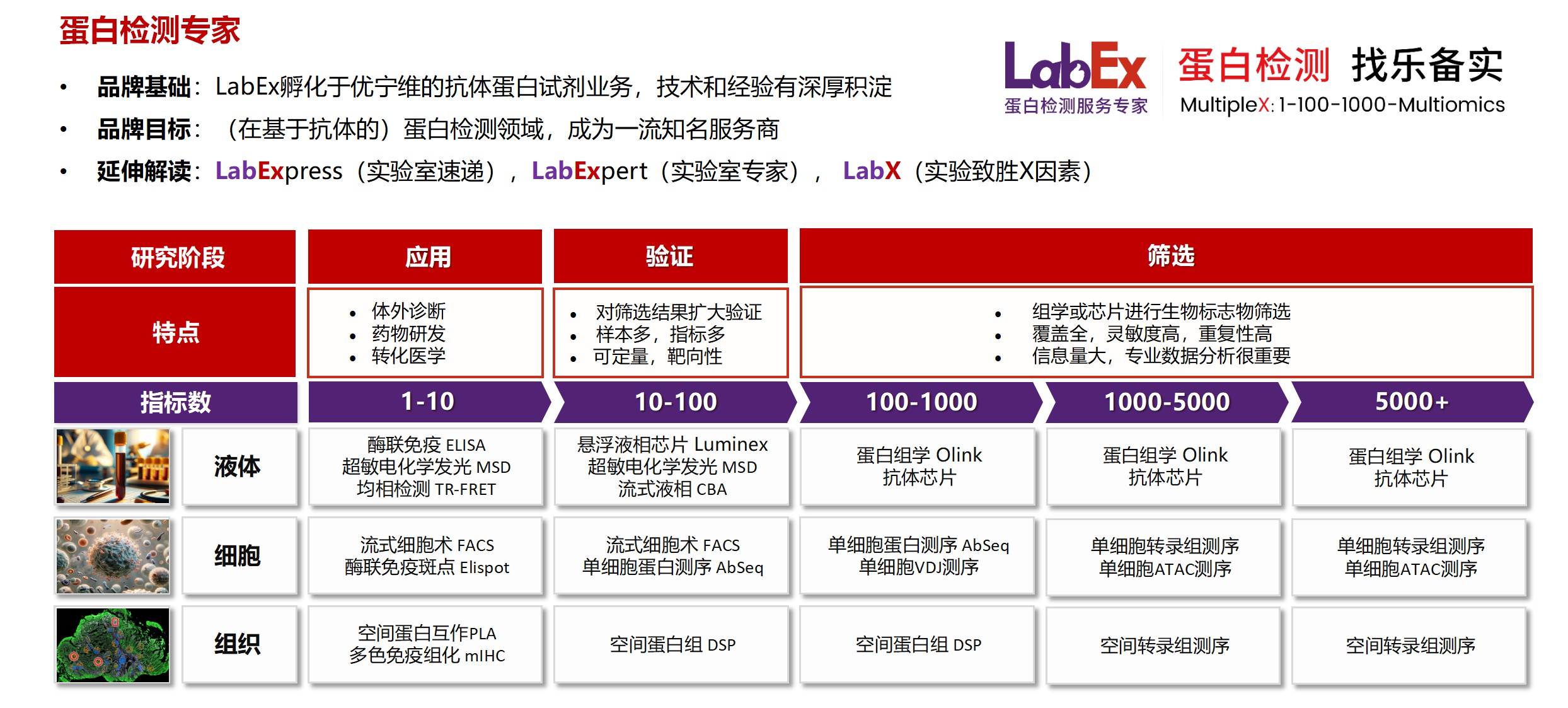
乐备实(上海优宁维生物科技股份有限公司旗下全资子公司),是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


