Cytokine release syndrome (CRS) is an undesired immune reaction that may cause dangerous side effects after the administration of novel biological therapies. In vitro cytokine release assays (CRA) are used for preclinical safety assessment prior to first-in-man dose administration of therapeutic monoclonal antibodies (mAbs). A variety of CRA platforms has been developed where the analysis of secreted cytokines is performed. Analysis of T cell activation markers is not performed routinely in CRA platforms and few studies have described intracellular cytokine levels after stimulation with therapeutic mAbs. In the present study, we performed a CRA using intracellular cytokine staining and assessment of extracellular T cell activation markers by flow cytometry. We used commercially available reference mAbs for the stimulation of peripheral blood mononuclear cells (PBMCs). We found that stimulation using solid phase (SP) dry coating with two different CD28 antibodies and muromonab-CD3 increased the percentage of IFN-ɣ + CD4+ and CD8+ T cells as well as of CD3-CD56+ NK cells compared to stimulation with antibodies in aqueous phase (AP). Expression of the T cell activation markers CD25 and CD69 on CD4+ and CD8+ T cells was also increased upon SP muromonab-CD3 stimulation. Using multiplex cytokine assessment, we showed that stimulation in AP using ANC28.1, CD28.2 and muromonab-CD3 led to an increase of IFN-ɣ, GM-CSF, TNF-α, and IL-2 secretion. Stimulation of PBMCs preincubated at high-density culture led to an increase in IFN-ɣ production but not in the expression of activation markers compared to low-density culture. Our findings demonstrated that flow cytometry analyses for assessing relevant T cell and NK cell markers may be used as a supplement to multiplex cytokine analysis in CRAs. The approach may be a valuable addition that enables a more precise description of the mechanisms leading to CRS.
Keywords:Cytokine release assay; Cytokine release syndrome; Flow cytometry.
Development of a flow cytometry-based potency assay for prediction of cytokine storms induced by biosimilar monoclonal antibodies
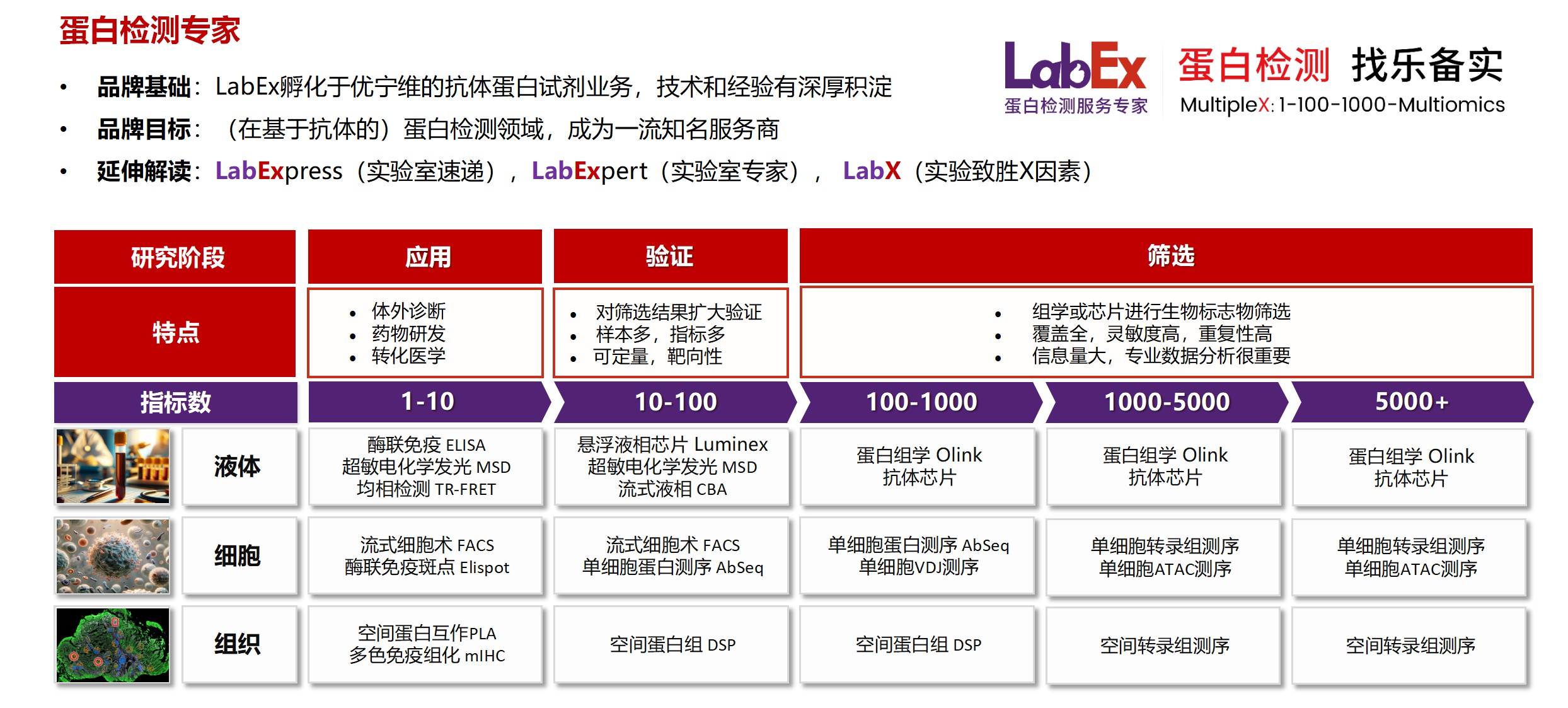
乐备实是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


