Single-cell multimodal analysis identifies common regulatory programs in synovial fibroblasts of rheumatoid arthritis patients and modeled TNF-driven arthritis
Background: Synovial fibroblasts (SFs) are specialized cells of the synovium that provide nutrients and lubricants for the proper function of diarthrodial joints. Recent evidence appreciates the contribution of SF heterogeneity in arthritic pathologies. However, the normal SF profiles and the molecular networks that govern the transition from homeostatic to arthritic SF heterogeneity remain poorly defined. Methods: We applied a combined analysis of single-cell (sc) transcriptomes and epigenomes (scRNA-seq and scATAC-seq) to SFs derived from naïve and hTNFtg mice (mice that overexpress human TNF, a murine model for rheumatoid arthritis), by employing the Seurat and ArchR packages. To identify the cellular differentiation lineages, we conducted velocity and trajectory analysis by combining state-of-the-art algorithms including scVelo, Slingshot, and PAGA. We integrated the transcriptomic and epigenomic data to infer gene regulatory networks using ArchR and custom-implemented algorithms. We performed a canonical correlation analysis-based integration of murine data with publicly available datasets from SFs of rheumatoid arthritis patients and sought to identify conserved gene regulatory networks by utilizing the SCENIC algorithm in the human arthritic scRNA-seq atlas. Results: By comparing SFs from healthy and hTNFtg mice, we revealed seven homeostatic and two disease-specific subsets of SFs. In healthy synovium, SFs function towards chondro- and osteogenesis, tissue repair, and immune surveillance. The development of arthritis leads to shrinkage of homeostatic SFs and favors the emergence of SF profiles marked by Dkk3 and Lrrc15 expression, functioning towards enhanced inflammatory responses and matrix catabolic processes. Lineage inference analysis indicated that specific Thy1+ SFs at the root of trajectories lead to the intermediate Thy1+/Dkk3+/Lrrc15+ SF states and culminate in a destructive and inflammatory Thy1- SF identity. We further uncovered epigenetically primed gene programs driving the expansion of these arthritic SFs, regulated by NFkB and new candidates, such as Runx1. Cross-species analysis of human/mouse arthritic SF data determined conserved regulatory and transcriptional networks. Conclusions: We revealed a dynamic SF landscape from health to arthritis providing a functional genomic blueprint to understand the joint pathophysiology and highlight the fibroblast-oriented therapeutic targets for combating chronic inflammatory and destructive arthritic disease.
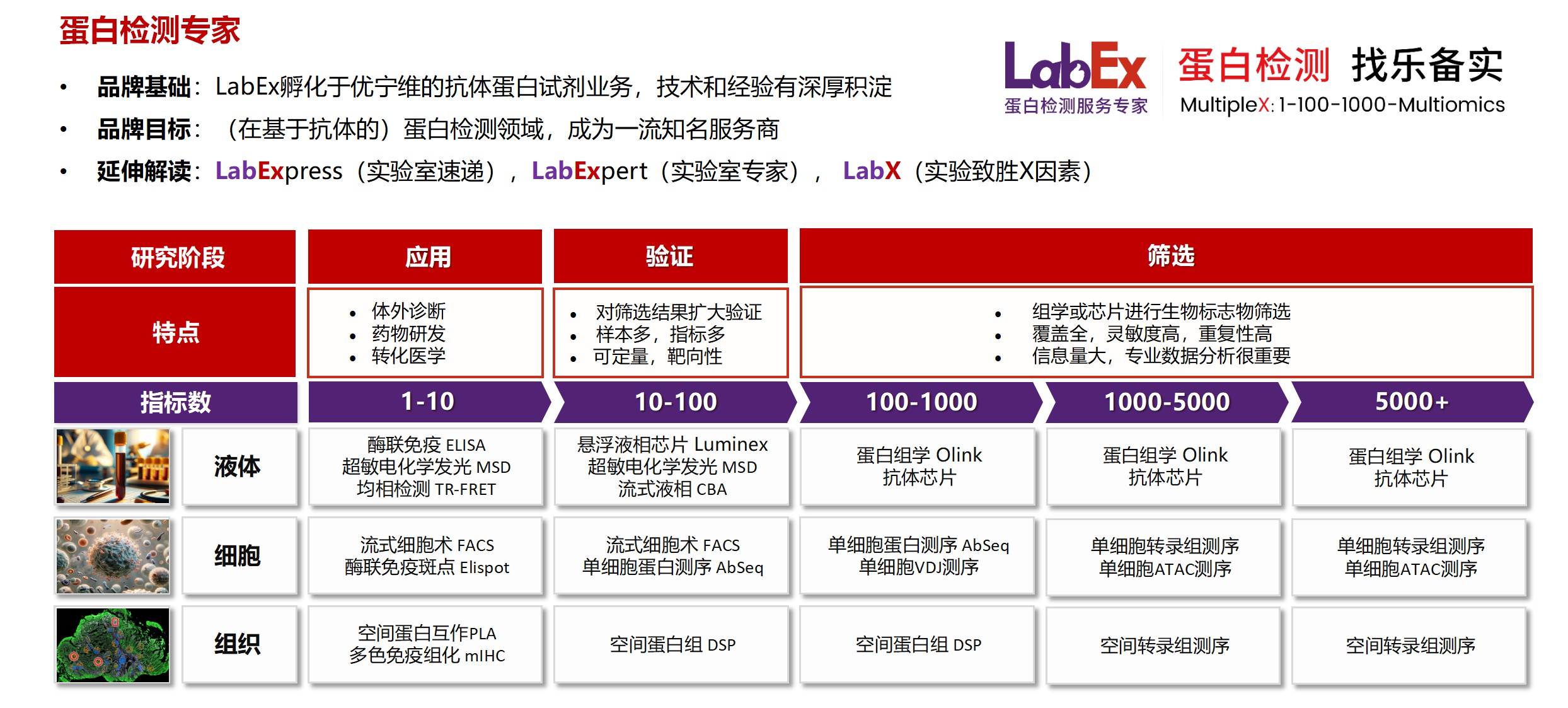
乐备实(上海优宁维生物科技股份有限公司旗下全资子公司),是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


