HS 3D-SeboSkin Model Enables the Preclinical Exploration of Therapeutic Candidates for Hidradenitis Suppurativa/Acne Inversa
Despite the rapid development in hidradenitis suppurativa (HS) research, the immediate introduction of potent therapeutic compounds in clinical trials and the lack of definitive outcome measures have led to the discontinuation of potential therapeutic compound studies. HS is a solely human disease, and therefore, the search for preclinical human models has been given priority. The 3D-SeboSkin model, a co-culture of human skin explants with human SZ95 sebocytes as a feeder layer, has been shown to prevent the rapid degeneration of human skin in culture and has been validated for HS preclinical studies. In this work, the HS 3D-SeboSkin model has been employed to characterize cellular and molecular effects of the EMA- and FDA-approved biologic adalimumab. Adalimumab, a tumor necrosis factor-α inhibitor, was shown to target inflammatory cells present in HS lesions, inducing a prominent anti-inflammatory response and contributing to tissue regeneration through a wound healing mechanism. Adalimumab inhibited the lesional tissue expression of TNF-α, IL-3, IL-15, and MCP-3 and downregulated the secretion of IL-1α, IL-5, RANTES, MCP-2, TNF-α, TNF-β, TGF-β, and IFN-γ. In contrast, IL-6 was stimulated. The compound failed to modify abnormal epithelial cell differentiation present in the HS lesions. Patients with Hurley stage II lesions exhibited stronger expression of autophagy proteins in perilesional than in lesional skin. Adalimumab modified the levels of the pro-apoptotic proteins LC3A, LC3B, and p62 in an individual, patient-dependent manner. Finally, adalimumab did not modify the NFκB signal proteins in SZ95 sebocytes and NHK-19 keratinocytes, used to study this specific pathway. The administration of the validated HS 3D-SeboSkin model in ex vivo studies prior to clinical trials could elucidate the individual pathogenetic targets of therapeutic candidates and, therefore, increase the success rates of clinical studies, minimizing HS drug development costs.Keywords:3D-SeboSkin; NFκB; SZ95 sebaceous gland cell line; adalimumab; autophagy; cytokines; ex vivo; hidradenitis suppurativa; proteins.
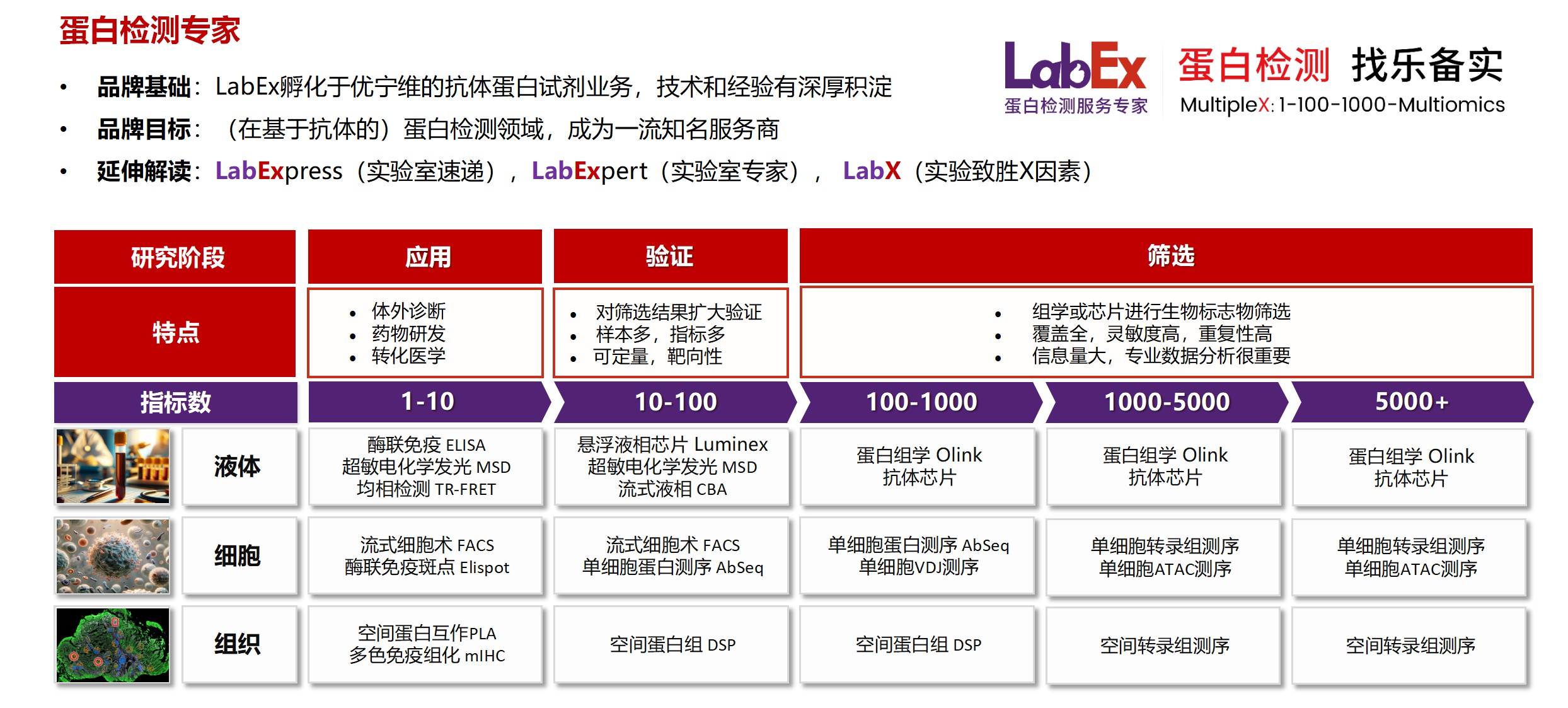
乐备实是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


