Purpose:Certain factors such as instrumental and sample processing errors may contribute to variability of ocular biofluid samples when they are run as replicates with multiplex assays. There is a paucity of literature on the variability of replicates in multiplex assays. This study aims to evaluate whether there is significant variability in replicate analyses of multiplex assays.
Methods:A total of 152 human ocular biofluid samples (51 aqueous humor and 101 vitreous) were collected and assayed for 27 cytokine biomarker concentrations (pg/mL). Samples were evaluated as replicates (duplicate analysis) at four different time points. Statistical methods including paired samples t-test, 3-way ANOVA, intraclass correlation coefficient (ICC; <0.5-0.75=poor-moderate, 0.75->0.90 =good-excellent reliability), and coefficients of variation (CV) were employed to evaluate for statistical significance, with Bonferroni corrected P=0.002.
Results:Among the 4104 biomarker replicate assays for aqueous humor and vitreous, two analytes (PDGF-BB and IL-7) had a statistically significant difference between the sampled concentrations of the replicates in vitreous samples (mean (diff)=2.05, P<0.001, mean (diff)=1.56, P<0.001, respectively). Majority of the ICC values fell within the good-excellent range (86% of samples) with a minority falling in the poor-moderate range (14% of samples). More variability was noted in the vitreous humour, with five analytes (IL-2, IL-10, IL-12(p70), IL-13, IL-17) demonstrating an average ICC of less than 0.5. The CV calculated for each set of replicates suggested that 93% of replicates had an acceptable level of quantitative assay variability (CV<20%).
Conclusion:This study demonstrates that the analysis of most biomarkers in ocular fluids may not require the use of replicates. However, certain analytes such as PDGF-BB and IL-7 may require the use of replicates to ensure reliable results. Caution should be taken when applying these findings to other laboratory settings as our study was conducted by an experienced technician using a standardized protocol. In less standardized settings, replicates may be required in order to ensure accuracy of results. These findings may guide researchers with the design of their studies on ophthalmic biomarker analysis.
Keywords:aqueous; assay; biomarkers; cytokines; vitreous.
Variability of Replicates of Intraocular Inflammatory Biomarkers in Ocular Fluid Samples Analyzed with Multiplex Assays
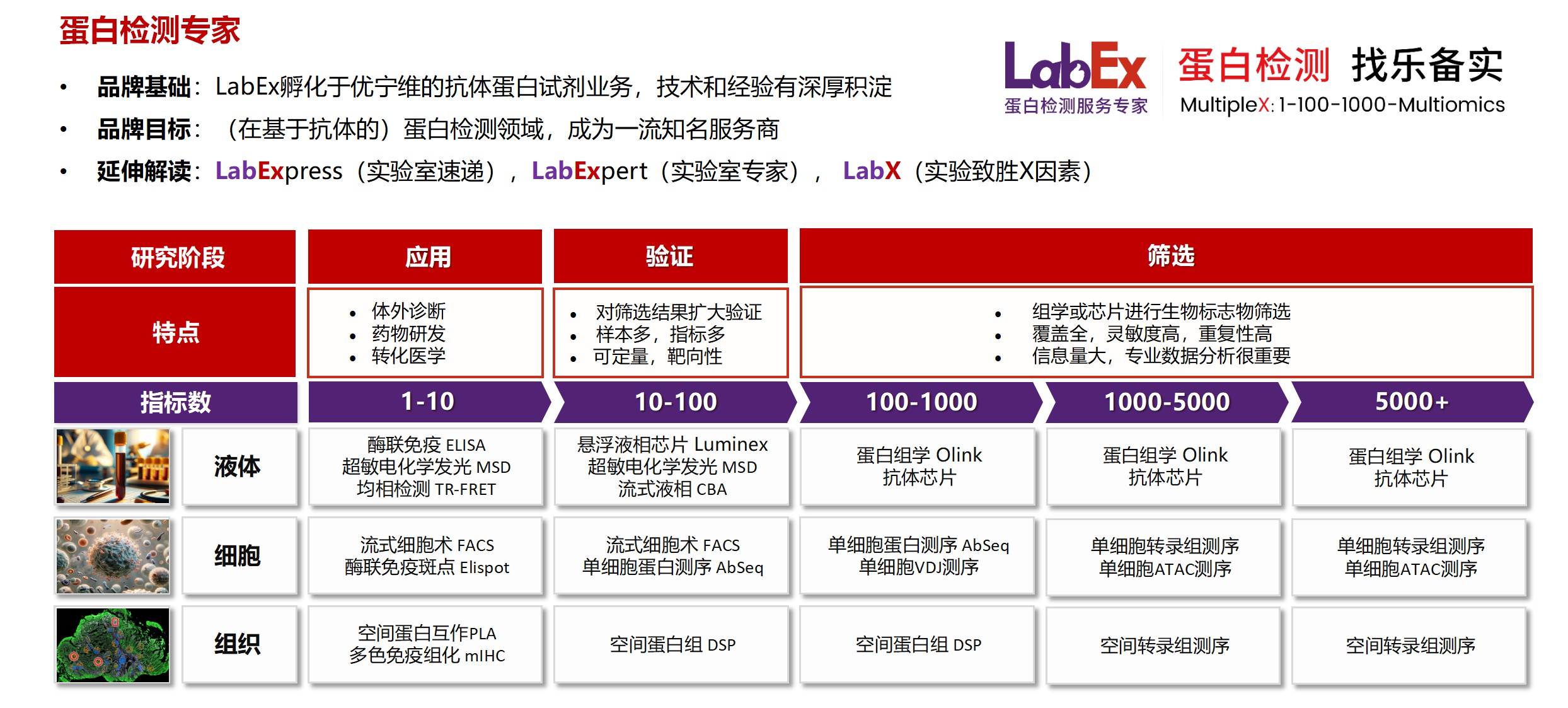
乐备实(上海优宁维生物科技股份有限公司旗下全资子公司),是国内专注于提供高质量蛋白检测以及组学分析服务的实验服务专家,自2018年成立以来,乐备实不断寻求突破,公司的服务技术平台已扩展到单细胞测序、空间多组学、流式检测、超敏电化学发光、Luminex多因子检测、抗体芯片、PCR Array、ELISA、Elispot、PLA蛋白互作、多色免疫组化、DSP空间多组学等30多个,建立起了一套涵盖基因、蛋白、细胞以及组织水平实验的完整检测体系。
我们可提供从样本运输、储存管理、样本制备、样本检测到检测数据分析的全流程服务。凭借严格的实验室管理流程、标准化实验室操作、原始数据储存体系以及实验项目管理系统,已经为超过3000家客户单位提供服务,年检测样本超过100万,受到了广大客户的信任与支持。
声明:本篇文章在创作中部分采用了人工智能辅助。如有任何内容涉及版权或知识产权问题,敬请告知,我们承诺将在第一时间核实并撤下。
详见LabEx网站(
www.u-labex.com)或来电咨询!
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
基因水平:PCR Array、RT-PCR、PCR、单细胞测序
蛋白水平:MSD、Luminex、CBA、Elispot、Antibody Array、ELISA、Sengenics
细胞水平:细胞染色、细胞分选、细胞培养、细胞功能
组织水平:空间多组学、多重荧光免疫组化、免疫组化、免疫荧光
数据分析:流式数据分析、组化数据分析、多因子数据分析
联系电话:4001619919
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家
联系邮箱:labex-mkt@u-labex.com
公众平台:蛋白检测服务专家

本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。










 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


