Chordoma recruits and polarizes tumor-associated macrophages via secreting CCL5 to promote malignant progression
Sarcoma,巨噬细胞,脊索瘤,CCL5 LabEX支持文献- J Immunother Cancer
- 2023
- 10.6
- 2023 Apr;11(4):e006808
- Human
- Luminex
- 生物标志物
- 细胞培养上清
- 生物标志物
- 巨噬细胞
- 脊索瘤
- β-NGF,CTACK/CCL27,Eotaxin/CCL11,FGF-basic,G-CSF,GM-CSF,GRO-α (Gro-a/KC/CXCL1),HGF,IFN-α2,IFN-γ,IL-1α,IL-1Rα,IL-2Rα,IL-1β,IL-2,IL-3,IL-4,IL-5,IL-6,IL-7,IL-8/CXCL8,IL-9,IL-10,IL-12(p40),IL-12(p70),IL-13,IL-15,IL-16,IL-17A,IL-18,IP-10/CXCL10,LIF,M-CSF,MCP-1/CCL2,MCP-3/CCL7,MIG,MIP-1α/CCL3,MIP-1β,MIF,PDGF-BB,RANTES,SCF,SCGF-β,SDF-1α,TRAIL,TNF-α,TNF-β,VEGF-A
- doi: 10.1136/jitc-2023-006808
相关货号
Abstract
Background: Chordoma is an extremely rare, locally aggressive malignant bone tumor originating from undifferentiated embryonic remnants. There are no effective therapeutic strategies for chordoma. Herein, we aimed to explore cellular interactions within the chordoma immune microenvironment and provide new therapeutic targets.
Methods: Spectrum flow cytometry and multiplex immunofluorescence (IF) staining were used to investigate the immune microenvironment of chordoma. Cell Counting Kit-8, Edu, clone formation, Transwell, and healing assays were used to validate tumor functions. Flow cytometry and Transwell assays were used to analyze macrophage phenotype and chemotaxis alterations. Immunohistochemistry, IF, western blot, PCR, and ELISA assays were used to analyze molecular expression. An organoid model and a xenograft mouse model were constructed to investigate the efficacy of maraviroc (MVC).
Results: The chordoma immune microenvironment landscape was characterized, and we observed that chordoma exhibits a typical immune exclusion phenotype. However, macrophages infiltrating the tumor zone were also noted. Through functional assays, we demonstrated that chordoma-secreted CCL5 significantly promoted malignancy progression, macrophage recruitment, and M2 polarization. In turn, M2 macrophages markedly enhanced the proliferation, invasion, and migration viability of chordoma. CCL5 knockdown and MVC (CCL5/CCR5 inhibitor) treatment both significantly inhibited chordoma malignant progression and M2 macrophage polarization. We established chordoma patient-derived organoids, wherein MVC exhibited antitumor effects, especially in patient 4, with robust killing effect. MVC inhibits chordoma growth and lung metastasis in vivo.
Conclusions: Our study implicates that the CCL5-CCR5 axis plays an important role in the malignant progression of chordoma and the regulation of macrophages, and that the CCL5-CCR5 axis is a potential therapeutic target in chordoma.
Keywords: Sarcoma.
LabEx Luminex平台助力脊索瘤的肿瘤微环境研究
本周为大家带来的文献为发表于J Immunother Cancer. (IF:10.9)的” Chordoma recruits and polarizes tumor-associated macrophages via secreting CCL5 to promote malignant progression”。本文使用了LabEx提供的Luminex检测服务。
本研究深入探讨了脊索瘤(chordoma)的肿瘤微环境(TIME),特别是巨噬细胞在肿瘤发展中的作用。通过使用人类脊索瘤细胞系和单核细胞系,研究发现脊索瘤微环境中存在M2型巨噬细胞,这些巨噬细胞可能通过促进肿瘤细胞的增殖和存活来影响肿瘤的发展。此外,研究还揭示了M2型巨噬细胞(CD206阳性)比M1型(CD86阳性)更为丰富,是最主要的肿瘤浸润免疫抑制细胞类型。通过多重免疫荧光染色技术,研究分析了T细胞、B细胞、NK细胞和巨噬细胞在脊索瘤组织中的浸润情况,发现大量免疫细胞浸润在脊索瘤的纤维隔膜中。这些发现为理解脊索瘤的免疫微环境提供了深入的视角,并为未来的治疗策略设计提供了重要信息。研究还探讨了脊索瘤与M2型巨噬细胞之间的相互作用及其对肿瘤进展的影响。研究发现,脊索瘤呈现出一种免疫排斥表型,其中大量免疫细胞存在于肿瘤间质中,但被排除在肿瘤本身之外。T细胞和巨噬细胞是主要的浸润细胞类型,其中CD3-阳性T细胞是最丰富的亚型。M2巨噬细胞在肿瘤区域中的分布比IRF-阳性M1巨噬细胞更丰富,并且能够打破物理屏障,积聚在肿瘤细胞附近。这些发现表明M2巨噬细胞在脊索瘤的恶性进展中可能发挥重要作用。总之,这些研究为我们深入了解癌症的发生机制和开发新的治疗策略提供了宝贵的信息。
LabEx提供的Lumienx检测服务:
为了确定脊索瘤与巨噬细胞相互作用的关键介质,研究者通过 Luminex 检测法检测了脊索瘤或巨噬细胞的条件培养基以及共培养系统的培养上清中的48种细胞因子。
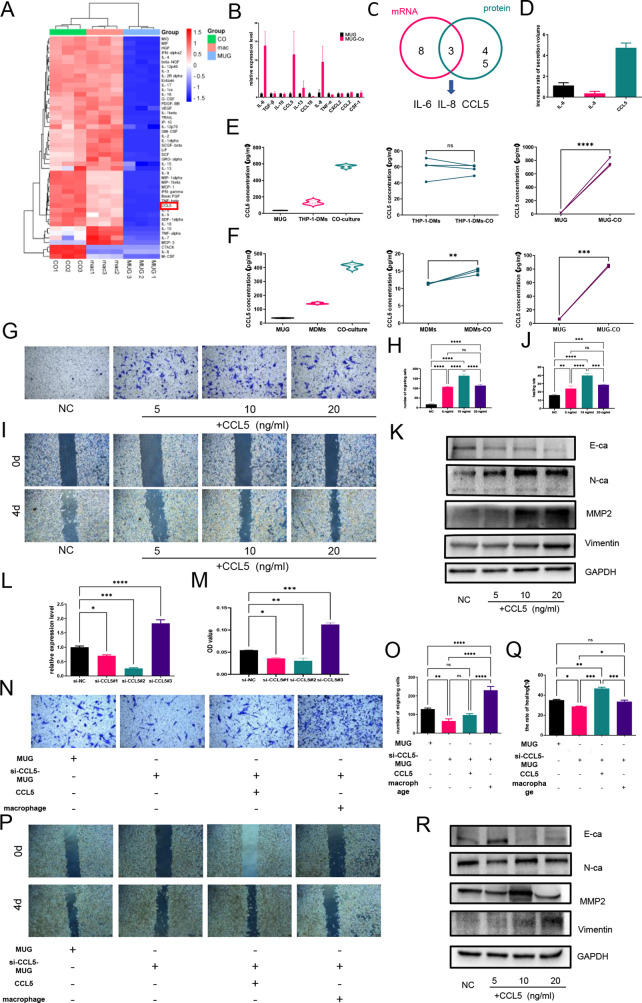
脊索瘤分泌的 CCL5 是参与脊索瘤细胞与巨噬细胞之间细胞通讯的关键细胞因子。
重要发现:
- 脊索瘤的肿瘤免疫微环境(TIME)仍然鲜为人知,阻碍了治疗目标的确定。
- T细胞是主要的浸润免疫细胞,而M2巨噬细胞数量丰富,并能穿透肿瘤屏障。
- 与单细胞RNA测序和多重免疫荧光等新技术不同的是,H&E染色等传统方法对脊索瘤的免疫情况了解有限。
- 细胞因子分析发现,CCL5 是脊索瘤细胞和巨噬细胞之间的关键介质,能促进肿瘤侵袭和巨噬细胞极化。
- 临床相关性显示,CCL5/CCR5的表达与脊索瘤的复发有关,表明其在肿瘤侵袭和巨噬细胞浸润中的作用。
- 用CCR5拮抗剂MVC阻断CCL5/CCR5信号传导可抑制脊索瘤的进展和巨噬细胞的极化。
- 类器官培养模型证实了MVC的疗效,为脊索瘤的治疗提供了一种很有前景的方法。
- 不足之处包括需要在人源化小鼠模型中进行进一步分析,并加深对巨噬细胞表型和空间特征的了解。
本网站销售的所有产品及服务均不得用于人类或动物之临床诊断或治疗,仅可用于工业或者科研等非医疗目的。











 沪公网安备31011502400759号
沪公网安备31011502400759号
 营业执照(三证合一)
营业执照(三证合一)


